Microorganisms for energy – does it work? And how could this be connected with CO2 conversion? Microorganisms particularly gained interest in carbon capture and utilization research due to the ability to convert CO2 to a broad range of possible valuable products and fuels. Application of such microorganisms has become highly attractive as several different strains of pure as well as mixed cultures of microorganisms are suitable for application in biofuel and biochemical generation.
Microbial electrochemical technologies (METs) combine microorganisms and electrodes. Thereby, electrodes serve as electron acceptor or donor in a microbial metabolism. For nearly one decade scientists engaged intensive with METs, including microbial fuel cells (MFCs) and microbial electrosynthesis (MES).
This process appears as an attractive option for CO2 conversion due to mild reaction conditions (ambient pressure and moderate temperatures) as well as low-cost and self-sustaining biocatalysts. (Kondratenko et al., 2013) (Wang and Ren, 2013).
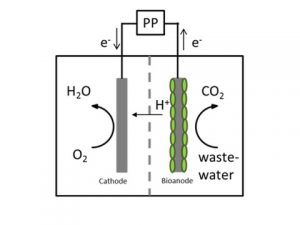
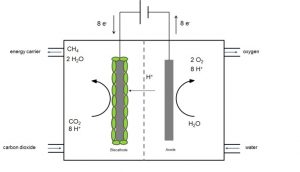
Microbial electrosynthesis has a great potential to directly produce easy and safe manageable energy carriers like alcohols, methane and organic acids from electricity and carbon dioxide or waste organics. There are two different ways how microorganisms catalyze the production of fuels or chemicals from CO2 in a microbial electrosynthesis cell:
- Firstly direct extracellular electron transfer – electrons are directly taken up from the electrode
- Secondly indirect extracellular electron transfer. For the indirect mechanism, for example hydrogen can be produced (bio)electrochemically directly at the cathode surface and then hydrogen and CO2 are transformed to the desired product.
The various mechanisms for electron transfer from a cathode to microbes are not fully understood, but are described in an increasing number of publications. For the direct electron transfer, electron-shuttling via cytochromes on the outer membrane of the cell is proposed (Yang et al., 2012). The involved c-type cytochrome electron transfer chains can further be assisted by hydrogenases (Rosenbaum et al., 2011). Direct electron transfer furthermore can be performed with other redox proteins, like Rnf complexes (a membrane-bound NADH:ferredoxin oxidoreductase) in Clostridium ljungdahlii or Rubredoxin in Clostridium pasteurianum (Choi and Sang, 2016).
Research in this area is an early stage and there is still intensive work required for optimization to possible large scale applications. At present, one key factor for economic success appears to lowering both the costs of electrode and associated materials (membranes, current collectors, etc.) as well as energy losses.
Stefanie Schlager, Anita Fuchsbauer, Marianne Haberbauer, Helmut Neugebauer and Niyazi Serdar Sariciftci: J. Mater. Chem. A, 2017, 5, 2429
Stefanie Schlager, Marianne Haberbauer, Anita Fuchsbauer, Christine Hemmelmair, Liviu Mihai Dumitru, Gabriele Hinterberger, Helmut Neugebauer, and Niyazi Serdar Sariciftci: ChemSusChem 2017, 10, 226
Marianne Haberbauer, Christine Hemmelmair, Sophie Thallner, Wolfgang Schnitzhofer: CO2-conversion to Acids and Alcohols by Microbial Electrosynthesis; Poster at ISMET 6, 03.- 06. October 2017, Lisbon, Portugal
Picture credits: Shutterstock
